CAPMUL® GDB EP/NF in Pharmaceutical Unit Operations
CAPMUL GDB EP/NF is a highly-functional, highly-reproducible solid lipid excipient manufactured for use in pharmaceutical formulations. CAPMUL GDB EP/NF meets the specifications of both the EP and NF for glyceryl dibehenate.
CAPMUL GDB EP/NF is a versatile excipient that is readily employed in a multitude of pharmaceutical manufacturing unit operations to providing varying functionalities, including multi-particulate diluent, sustained-release coating, matrix sustained-release excipient, tablet lubricant, protective coating, and taste-masking excipient.
CAPMUL GDB EP/NF is readily employed in the following pharmaceutical unit operations:
- direct compression tableting
- granulation/hot-melt extrusion
- spray congealing
- spray coating
- solid lipid nanoparticle manufacturing
Direct compression tableting
CAPMUL GDB EP/NF is generally employed in direct-compression tableting as a sustained- release matrix excipient or as a boundary lubricant. In both cases, the CAPMUL GDB EP/NF is added directly into the tablet formulation by blending with other formulation ingredients, employing typical industry mixing equipment. The physiochemical properties, shape, and particle size of CAPMUL GDB EP/NF allow the excipient to be readily dispersed in a uniform fashion, in order to produce a randomized mix with the remaining tableting ingredients upon blending.
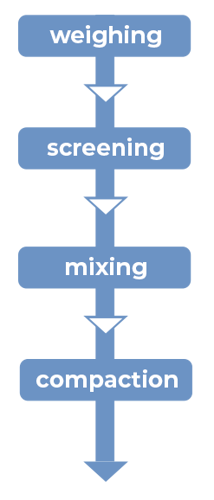
Figure 1: Manufacturing unit operations for direct compression with CAPMUL GDB EP/NF
With regard to sustained release, the concentration of CAPMUL GDB EP/NF within each respective formulation will control the release of the active. Generally, as more CAPMUL GDB EP/NF is added to the formulation, the slower the dissolution rate. The primary mechanism of active dissolution out of a sustained release tablet manufactured with CAPMUL GDB EP/NF is diffusion. As such, selection of diluent excipients will affect the active dissolution rate, with water soluble diluents, such as lactose and mannitol increasing dissolution rate, as compared with water insoluble excipients such as dibasic calcium phosphate, which reduce dissolution rate, comparatively.
CAPMUL GDB EP/NF is a boundary lubricant, indicating it does not shear and spread over tablet ingredient surfaces upon blending. In contrast, the CAPMUL GDB EP/NF diffuses uniformly throughout the tablet blend upon mixing. In this way, at points of contact between the CAPMUL GDB EP/NF and tablet machinery, such as the die walls and punches, the CAPMUL GDB EP/NF provides boundary lubrication, reducing sticking and lowering ejection force.
Granulation
CAPMUL GDB EP/NF can be employed in aqueous, solvent-based, and hot-melt granulations. In both aqueous and solvent-based granulations, CAPMUL GDB EP/NF is typically employed as a sustained-release excipient. CAPMUL GDB EP/NF can be added as part of the granulation unit operation, or it can be added after granulation prior to direct compression as lubricant for direct compression of the granules. Active release can be adjusted by adjusting the concentration of CAPMUL GDB EP/NF in the granule formulation.
Figure 2: Manufacturing unit operations for granulations with CAPMUL GDB EP/NF
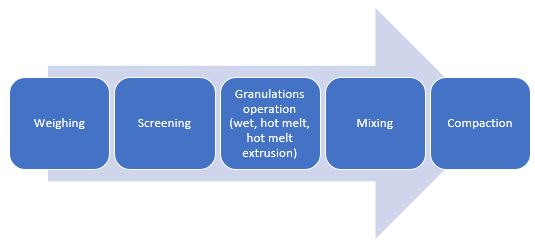
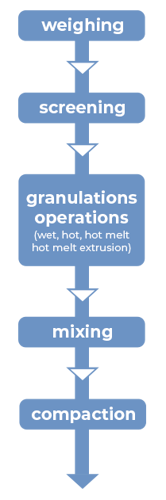
In hot-melt granulations, including hot-melt granulations manufactured by hot-melt extrusion, CAPMUL GDB EP/NF can act as a binder, a taste-masked coating, a protective coating, a diluent, and/or a sustained-release excipient. For hot melt extrusion, the CAPMUL GDB EP/NF is blended with other formulation excipients in concentrations suitable for application purposes and this mixture is introduced into a hot-melt extruder. Subsequently, the granulation or extrudate is collected for further processing. CAPMUL GDB EP/NF can be employed in other hot-melt unit operations, such as high shear hot-melt granulation which employs a jacketed hi-shear granulator or in hot melt fluid bed granulations where CAPMUL GDB EP/NF is sprayed onto a fluidized bed of active and other granulation excipients. Generally, the extrudates or granules from these operations are sized and then blended with other tableting excipients and then compacted on rotary tableting equipment.
Spray congealing/drying
CAPMUL GDB EP/NF is a solid lipid excipient at room temperature. As such, it is readily employable in spray congealing unit operations. CAPMUL GDB EP/NF can be molten with lipid soluble excipients to form a molten mixture, which can be sprayed into lipid/active multi-particulates for subsequent processing (Figure 3). Additionally, lipid insoluble actives can be uniformly suspended in CAPMUL GDB EP/NF. This lipid/active suspension can be sprayed to form multi-particulates for subsequent processing. In a spray congealing unit operation, CAPMUL GDB EP/NF can be employed primarily as a sustained-release excipient, a taste-masking excipient, a multi-particulate diluent, or a protective coating against chemical reactions, such as oxidation or hydrolysis.
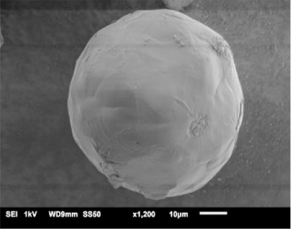
Figure 3: Ibuprofen and glyceryl behenate solid lipid nanoparticle (1)
Spray Coating
CAPMUL GDB EP/NF can be employed as a functional coating for hot-melt, fluid-bed unit operations. CAPMUL GDB EP/NF alone or with other ingredients can be sprayed in molten form onto a fluidized bed of raw materials, including actives and other excipients. This unit operation generates coated multi-particulates for subsequent processing. In this application, CAPMUL GDB EP/NF can be employed as a sustained release coating, a protective coating, or taste-masking coating.
Solid lipid nanoparitcle manufacturing (2)
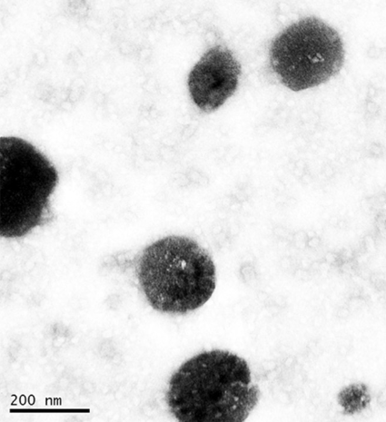
CAPMUL GDB EP/NF mixed with select surfactants can form spontaneous self-emulsifying active-carrying isotropic systems when homogenized or stirred in hot water. Upon cooling and solvent evaporation, these micellar systems form active carrying solid lipid nanoparticles (Figure 4), which can be employed in the further processing of a dosage forms. This type of processing allows for specialized routes of administration, such as inhalation. Additionally, active contained in the core of the lipid nanoparticle is protected from oxidation and hydrolysis events.
Figure 4: Rifabutin containing solid lipid nanoparticles manufactured with glyceryl dibehenate
REQUEST A SAMPLE
References
- Priscilla Chui Hong Wong, Paul Wan Sia Heng & Lai Wah Chan (2016) A study on the solid state characteristics of spray-congealed glyceryl dibehenate solid lipid microparticles containing ibuprofen, Drug Development and Industrial Pharmacy, 42:3, 364-377
- Gaspar D. et al (2016) Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: Physicochemical and in vitro studies, International Journal of Pharmaceutics, 497, 199-209.






