Manufacture Durable Tablets with a Direct Compression Lubricant, is that even possible?
Dr. John Tillotson, Technical Business Director ABITEC If you are looking for increased tablet durability, ease of use, great economy, as well as efficient tablet lubrication on your next direct compression tableting development, think Sterotex® NF, the lubricating dry-binder from ABITEC.
Often you find yourself manufacturing a tablet by direct compression, but the lubricant disrupts tablet bonding, leading to a decrease in tablet durability as measured by a loss in tablet hardness. This is an all too familiar story, especially with tablets which need to carry large amounts of active material, which tends to be uncompressible. ABITEC recently compared its lubricating dry-binder Sterotex® NF (hydrogenated vegetable oil type I) to industry leading dry binders copovidone (CP) and micronized hydroxypropyl cellulose (mHPC) for tablet hardness in direct-compression tableting with surprising results.
You may not be aware of it because ABITEC is well known for manufacturing highly-reproducible lipids for self-emulsifying drug delivery systems for the both the pharmaceutical and nutraceutical industries, but ABITEC also manufactures highly-reproducible ingredients for direct compression tableting. One of our most interesting ingredients is a direct-compression tablet lubricant that also serves as an effective and economical direct-compression dry binder to improve tablet durability. This exciting excipient is Sterotex® NF.


You may not have seen it, but recently ABITEC compared its lubricating dry binder Sterotex® NF with CP and mHPC to study the effect of dry binder type, concentration and tablet press compression force on tablet durability as measured by tablet hardness.
Table 1: Tablet formulations
|
Designation |
Acetaminophen |
Spray-dried mannitol |
Sterotex® NF |
CP |
mHPC |
Mg stearate |
|
0% APAP |
0.0 |
98.0 |
0.0 |
0.0 |
0.0 |
2.0 |
|
20% APAP |
20.0 |
78.0 |
0.0 |
0.0 |
0.0 |
2.0 |
|
Sterotex NF |
20.0 |
73.0 |
5.0 |
0.0 |
0.0 |
2.0 |
|
mHPC |
20.0 |
73.0 |
0.0 |
0.0 |
5.0 |
2.0 |
|
CP |
20.0 |
73.0 |
0.0 |
5.0 |
0.0 |
2.0 |
Testing was done employing spray-dried mannitol a brittle fracturing material as a primary diluent and 5.0% or 10.0% of each dry binder was added, as well as 20.0% acetaminophen powder, which is nearly uncompressible, as a compression challenge. 1000mg tablets were manufactured on a Manesty rotary tablet press outfitted with 0.625” FFBE type “B” punches at a rotational velocity of 40rpm and a compression cycle dwell time of 11msec. Tablets were manufactured at 15 and 25kN of compression force. Collected tablets were measured for tablet hardness as a measure of durability.
Tablet Hardness (5.0% dry binder concentration) Tablet Hardness (10.0% dry binder concentration)
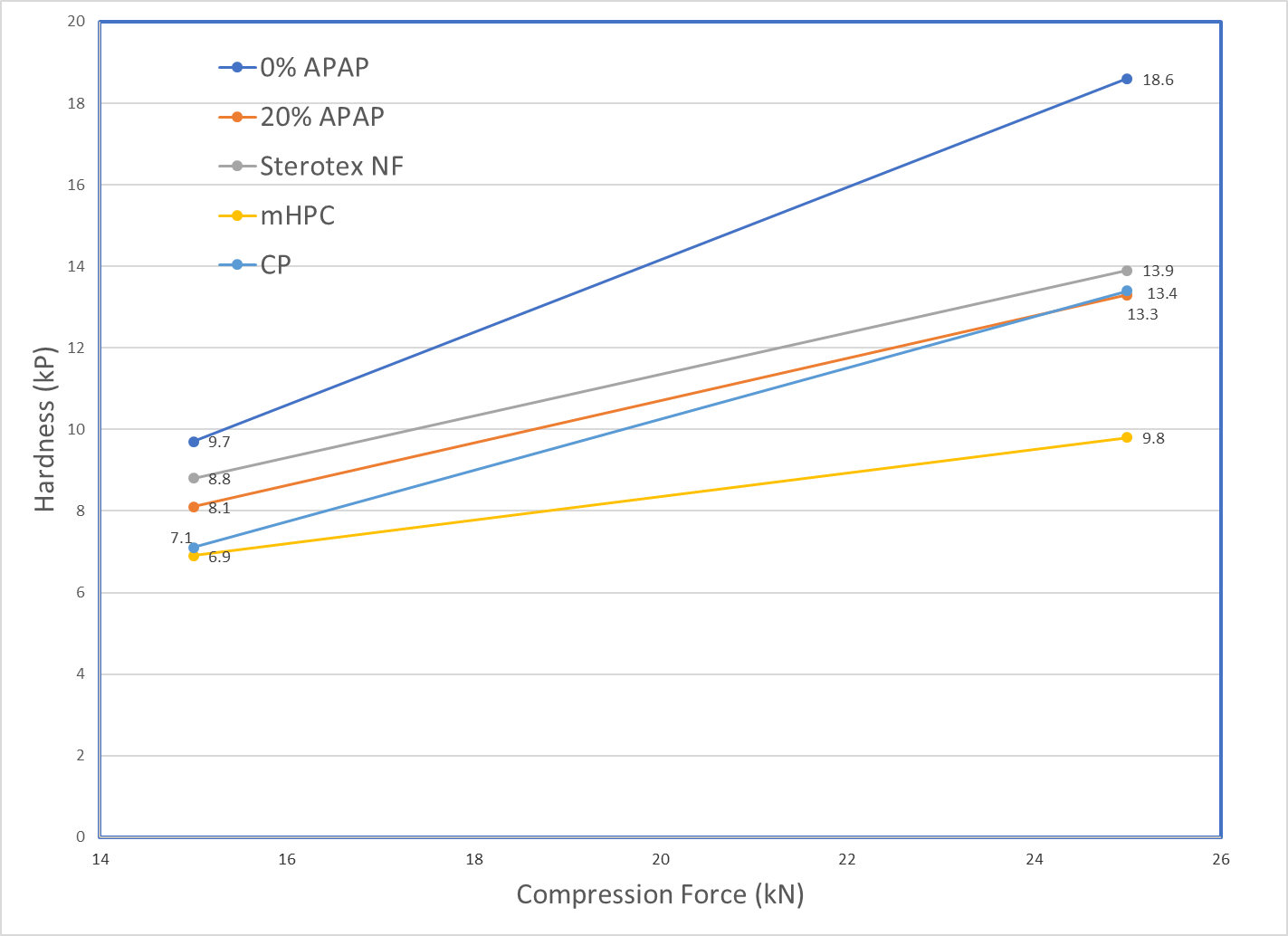
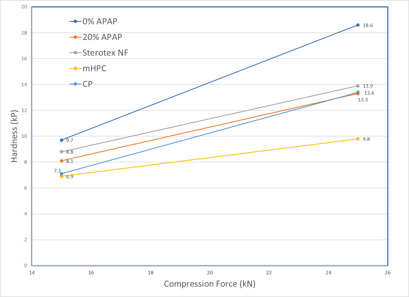
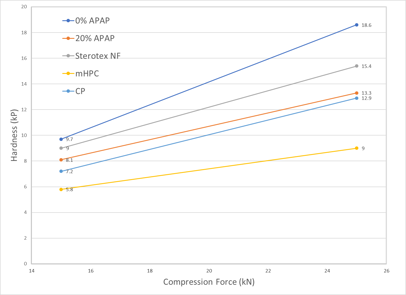
Sterotex® NF produced harder tablets than mHPC and CP at all dry binder concentrations and compression forces. This added hardness increases tablet dilution capacity and durability.
Sterotex® NF is a plastically deforming excipient at low yield pressures which exhibits little elastic recovery, and for this reason is ideal in applications as a dry binder. Sterotex® NF also acts as a lubricant and lowers ejection force, but as it exerts its lubricating effect in the free fraction, it does not prolong disintegration times. Finally, Sterotex® NF is easily added to tablet blends by direct dry mixing with industry standard blenders.
If you are looking for increased tablet durability, ease of use, great economy, as well as efficient tablet lubrication on your next direct compression tableting development, think Sterotex® NF, the lubricating dry-binder from ABITEC.
To Sample Sterotex NF Today visit our Sample Page!
STEROTEX® is a registered trademark of ABITEC Corporation. All information and statements given on this blog are believed to be accurate at the time of publication. However, neither ABITEC Corporation nor any of their affiliates make any representations or warranty with respect thereto, including, but not limited to, any results obtained in the processing of the products by customers or any third party. All information and statements are intended for persons having the required skill and know-how and do not relieve the customer or user from verifying the suitability of information and statements given for a specific purpose prior to use of the products. It is entirely the obligation of the customer or user to comply with applicable laws and regulations, and also with all patent or other intellectual property rights of third parties. ABITEC CORPORATION EXPRESSELY DISCLAIMS ANY REPRESENTATIONS OR WARRANTIES OF ANY KIND, WHETHER EXPRESSED OR IMPLIED, AS TO THE ACCURACY, CURRENCY, COMPLETENESS AND/OR THE MERCHANTABILITY OR FITNESS OF A PARTICULAR PURPOSE OF ANY INFORMATION CONTAINED ON THIS BLOG AND/OR PRODUCT DESCRIBED OR PROMOTED ON THIS BLOG, INCLUDING WARRANTIES WITH RESPECT TO INFRINGEMENT OF ANY PATENT, COPYRIGHT, OR OTHER RIGHTS OF A THIRD PARTY. We reserve the right to change product specifications and specified properties of the products without prior notice.

