Top 3 Reasons to Formulate Biologics with Lipids
Dr. Heath Bowers, Technical Business Director ABITEC The use of lipids in the formulation of biologics is increasing for obvious reasons. Each therapeutic faces its own set of challenges. Luckily for you, there are several classes of functional lipids that can assist in the development of your biologic ensuring a successful entry into the market.
Eleven of the top fifteen therapeutics sold in 2018 were biologics (proteins or peptides) and combined for a total revenue of approximately 86 billion dollars (USD) (1). The current supremacy of biologics lies in their high specificity towards one target, whereas small molecules can unintentionally bind to multiple targets. Consequently, biologics tend to elicit less side effects compared to small molecules. However, biologics face a host of challenges including poor long-term stability and oral bioavailability. One way the pharmaceutical industry has addressed these obstacles is by freeze-drying formulations and dosing biologics parenterally respectively. As a result, a trained medical professional must administer the biologic and strict adherence to aseptic manufacturing and packing must be practiced. It’s no wonder pharmaceutical scientist are beginning to utilize the power and ease of functional lipids for the delivery of biologics.
Reason #1: Protection of Biologics

Much like small molecule therapeutics, degradation of biologic drugs leads to a decrease in efficacy. Unlike small molecules however, the large size of biologics provides increased opportunity for enzymes, bile salts, and reactive oxygen species to degrade the therapeutic. Consequently, protecting biologics during delivery becomes an important goal during formulation, even more so if the dosage form is intended to be oral. Several lipid-based drug delivery systems (LBDD) have demonstrated an ability to protect peptides and plasmid DNA from degradation and thus improve their bioavailability (2, 3). Most notably, peptide emulsions using C8 and C10 fatty acid derivatives like Captex® 355 EP/NF and Capmul® MCM EP/NF reduced degradation of a peptide by trypsin and α-chymotrypsin (2). Alternatively, protection can be conferred via encapsulation within a solid lipid nanoparticle (SLNs). Long chain triglycerides like Sterotex® NF provide the best particle stability and therefore decrease the rate of therapeutic degradation (4).
Reason #2: Increased oral absorption

Absorption in the gastrointestinal tract occurs via paracellular or transcellular transport. Passive paracellular transport requires the molecule be small enough to pass through the tight junction gap while passive transcellular transport requires that molecule have low hydrogen bonding potential, be relatively small, and contain a certain lipophilicity. Biologics are large, polar, molecules that have a high potential for hydrogen bonding and therefore are precluded from passive paracellular or transcellular transport. Therefore, formulators need to enhance permeation through the tight junction gaps or cell membrane. Ester derivatives of C8 and C10 fatty acids like Capmul MCM C-10 and Capmul MCM C-8 EP/NF have been utilized to open the tight junction (5, 6) while long chain fatty acid derivatives like Capmul GMO-50 EP/NF have been shown to affect membrane fluidity and inhibit the Pgp efflux pump to increase transcellular absorption. Alternatively, formulators can increase transcellular absorption of molecules into the lymphatic system by formulating a LBDD system comprised of long chain triglycerides like Captex GTO (7).
Reason #3: Enhanced organ-specific biodistribution
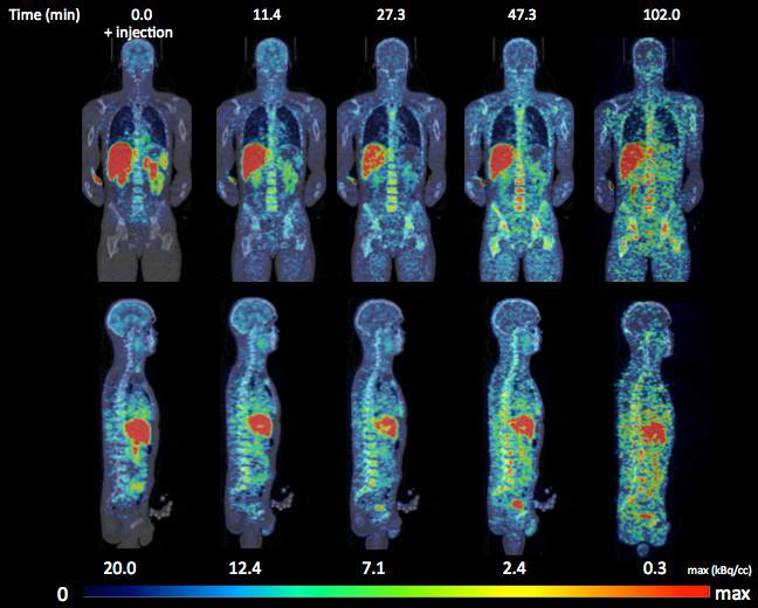
Since most biologics are dosed parenterally and therefore systemically, larger doses are required to achieve the desired therapeutic response. Consequently, severe side effects can occur such as renal impairment or ototoxicity such as the case with the glycopeptide Vancomycin (8). Delivering a molecule to only the intended organ or tissue is a goal every formulator strives for so that they can reduce the amount of the drug and still maintain efficacy. Recently, studies have demonstrated enhanced delivery of therapeutics to disease-specific areas using LBDD systems (9 - 13). SLNs made from lipids like Capmul GDB EP/NF have been used to enhance delivery to the brain for central nervous system disorders (11, 12). Even DNA shows a distinct delivery biodistribution depending on which lipids were used to make the SLNs (13). By delivering biologics in a targeted fashion using LBDD systems, therapeutics show improved potency and success in the pharmaceutical market. While lipids alone may not provide sufficient organ targeting, they can enhance delivery to a specified tissue.
Next steps for your biologic formulation…
The use of lipids in the formulation of biologics is increasing for obvious reasons. Each therapeutic faces its own set of challenges. Luckily for you, there are several classes of functional lipids that can assist in the development of your biologic ensuring a successful entry into the market.
Contact your local ABITEC representative for a consultation today
References:
- Philippidis, A. Gen Eng Biotech. Top 15 Best-selling drugs of 2018. 2019 Apr; 39 (4): 16 – 17
- Hintzen, F. et al. Inter J Pharmaceutics. In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin. 2014 Sep 10; 472 (1 – 2): 20 – 26
- Hauptstein, S. et al. Inter J Pharmaceutics. Self-nanoemulsifying drug delivery systems as novel approach for pDNA drug delivery. 2015 Jun 20; 487 (1 – 2): 25 – 31
- Chen, C. et al. Nanomedicine (Lond). Orally delivered salmon calcitonin-loaded solid lipid nanoparticles prepared by micelle-double emulsion method via the combined use of different solid lipids. 2013 Jul; 8 (7): 1085 – 1100
- Föger, F.A. et al. (2008) WO 2008/145728 Al. Bagsvaerd, Denmark. World Intellectual Property Organization
- Tuvia, S. et al. Pharm Res. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. 2014 Aug; 31 (8): 2010 – 2021
- Holm, R. et al. Eur J Pharm Sci. Comparison of total oral bioavailability and the lymphatic transport of halofantrine from three different unsaturated triglycerides in lymph-cannulated conscious rats. 2001 Dec; 14 (4): 331 – 337
- Valle, M. et al. Antimicrobial Agents and Chemotherapy. Pulmonary versus systemic delivery of antibiotics: comparison of vancomycin dispositions in the isolated rat lung. 2007 Oct; 51 (10): 3771–3774
- Surekha, R. et al. Inter J Pharmaceutical Clinical Res. An efficient encapsulation of thymoquinone using solid lipid nanoparticle for brain targeted drug delivery: physicochemical characterization, pharmacokinetics, and bio-distribution studies. 2016 Dec; 8 (12): 1616 – 1624
- Bondì, M.L. et al. Nanomedicine (Lond). Brain-targeted solid lipid nanoparticles containing riluzole: preparation, characterization and biodistribution. 2010 Jan; 5 (1):25 – 32
- Estella-Hermoso de Mendoza, A et al. J Control Release. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. 2011 Dec 20; 156 (3): 421 – 426
- Jose, S. et al. Int J Pharm. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. 2014 Oct 20; 474 (1-2): 6 – 13
- Mahato, R.I. et al. Hum Gene Ther. Biodistribution and gene expression of lipid/plasmid complexes after systemic administration. 1998 Sep 20; 9 (14): 2083 – 2099
CAPTEX® CAPMUL® and STEROTEX® are registered trademarks of ABITEC Corporation. All information and statements given on this blog are believed to be accurate at the time of publication. However, neither ABITEC Corporation nor any of their affiliates make any representations or warranty with respect thereto, including, but not limited to, any results obtained in the processing of the products by customers or any third party. All information and statements are intended for persons having the required skill and know-how and do not relieve the customer or user from verifying the suitability of information and statements given for a specific purpose prior to use of the products. It is entirely the obligation of the customer or user to comply with applicable laws and regulations, and also with all patent or other intellectual property rights of third parties. ABITEC CORPORATION EXPRESSELY DISCLAIMS ANY REPRESENTATIONS OR WARRANTIES OF ANY KIND, WHETHER EXPRESSED OR IMPLIED, AS TO THE ACCURACY, CURRENCY, COMPLETENESS AND/OR THE MERCHANTABILITY OR FITNESS OF A PARTICULAR PURPOSE OF ANY INFORMATION CONTAINED ON THIS BLOG AND/OR PRODUCT DESCRIBED OR PROMOTED ON THIS BLOG, INCLUDING WARRANTIES WITH RESPECT TO INFRINGEMENT OF ANY PATENT, COPYRIGHT, OR OTHER RIGHTS OF A THIRD PARTY. We reserve the right to change product specifications and specified properties of the products without prior notice.

